Annotated Registry of Trials in Cancer, Australia and New Zealand Ed. - Frequently Asked Questions
- What is ARTICANZ?
- ARTICANZ (Annotated Registry of Trials in Cancer, Australia/NZ Edition; ARTificial Intelligence-enhanced CANcer trial information database) is a free, open-information platform designed to provide comprehensive annotation of cancer drug trial information to oncologists/haematologists, researchers, trialists, patients, and different stakeholders in Australia and New Zealand.
- Why ARTICANZ?
- ARTICANZ is designed as an open-information platform providing comprehensive annotations on baseline information about cancer clinical trials, with the goal of supporting clinical decision-making for specialists, patients and researchers in Australia and New Zealand. ARTICANZ was created to overcome the challenge of finding relevant, knowledge-rich information required for cross-referrals and to support various digital applications downstream.
- What are the key features of ARTICANZ web interface?
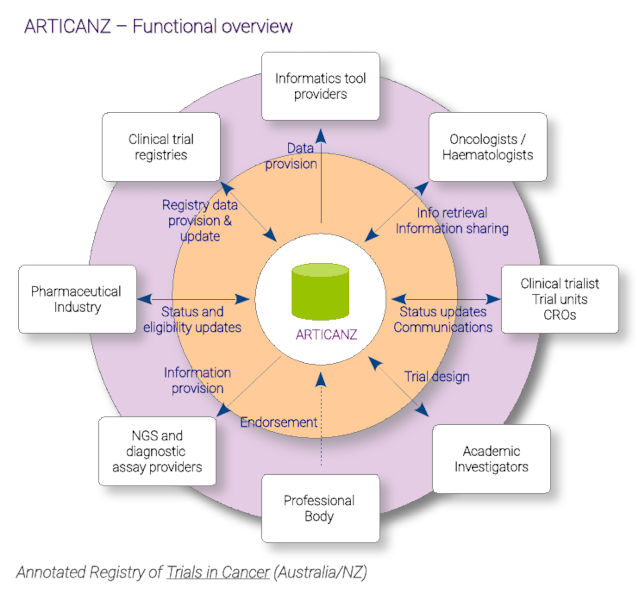
- ARTICANZ is a meta search engine that includes a comprehensive database with hierarchically categorised trial listings. Designed to meet oncologists' and haematologists' needs in the clinic, these listings include different "tags" that enable sophisticated searching to locate relevant trials efficiently. These include, but are not limited to, drug targets, drug classes, cancer types, operational status, and relevant eligibility criteria needed for initial trial pre-screening.
- How does ARTICANZ support research and clinical integration?
- The platform offers a tool set for researchers and clinicians, enhancing their ability to rapidly identify suitable trials:
- Specialist-level clinical informatics and bioinformatics annotations that are context relevant.
- Minimal relevant list and meta-data that supplements trial registry data.
- Novel strategies to keep up-to-date recruitment information.
- Can I use ARTICANZ data for commercial or research purposes?
-
- Data use
- Data from this website may be used freely, including in academic or commercial settings, but must follow the Creative Commons Attribution 4.0 International (CC BY 4.0) open source licence.
- ARTICANZ promotes the use of data and encourages data sharing between users and stakeholders to keep information relevant for clinicians, researchers, and patients. If there are discrepancies in data, we encourage sharing any updates or correct inaccuracies to benefit all users.
- Research and academic collaborations
- Yes, ARTICANZ welcomes academic research use and collaborations from clinical researchers and academic institutions.
- What is the long-term vision of ARTICANZ?
-
The long-term vision of ARTICANZ is to improve trial decision-making process by providing reliable and comprehensive data through:
- Open and independent - Enhance open information and knowledge sharing.
- Information sharing - Improve fairness and equality in knowledge access regarding cancer clinical trials.
- Rational information organization - To streamline information location for relevant oncology drug trials, from the requirment of specialist physicians.
- Regional collaboration - Promote trial participation and research data sharing in Australia and New Zealand.
- Platform enabling - Develop a low-cost, AI-enabled driven curation pipeline and IT platform to ensure sustainability.
- Decision support - Facilitates transparent streamlined clinical decision-support.
- Enhancing research intelligence - Allowing trialists and referrers to visualise the landscape of cancer trials in Australia and New Zealand.
- How frequently is ARTICANZ updated?
- ARTICANZ is updated regularly at least weekly (on Fridays) to ensure the most current trial information is made available through both web and data platforms. The database and annotations undergo systematic reviews by specialist oncologist/haematologist and updates to maintain accuracy and relevance of trial data, to the best of knowledge.
- How can I access ARTICANZ?
- ARTICANZ is freely accessible through its Web Platform, Data Downloads, and Application Programming Interfaces (API). For the web platform, users can perform search and browse trial information without requiring registration or login credentials.
- What types of cancer trials are included in ARTICANZ?
- ARTICANZ focuses on systematic categorisation of cancer drug trials conducted in Australia and New Zealand. The database includes various types of clinical trials, from early phase to late phase studies, across different cancer types and therapeutic approaches.
- Who funded ARTICANZ?
- ARTICANZ is currently self-funded and maintained by Dr. Frank Lin as an independent research initiative. To preserve our commitment to unbiased, transparent data — as a contributing component of the broader ecosystem of clinical decision-support (CDS) in oncology/haematology — we may accept funding from public sectors, government agencies, academic institutions, and philanthropic organisations in the future. Whilst we don't accept direct financial support from private industry to avoid potential conflicts of interest, we actively welcome and encourage collaborative partnerships with all stakeholders—including pharmaceutical companies, CROs, research organisations, and healthcare providers. These collaborations are essential for enhancing data accuracy, expanding coverage, and improving platform functionality. We believe that by working together while maintaining our independence, we can create a valuable resource for patients, clinicians, researchers, and all members of the cancer research community in Australia and New Zealand.
- How can I report errors or suggest improvements?
- Absolutely. ARTICANZ is designed to promote peer-to-peer open information sharing about cancer clinical trials. Clinicians, users, and relevant stakeholders are critical in the feeding back process. Please report any errors or suggest improvements by contacting the ARTICANZ team through the platform's feedback system. We welcome user input to help enhance the quality and utility of the database.
- How does ARTICANZ ensure data quality?
-
In addition, ARTICANZ maintains data quality through:
- Algorithmic backend for systematic curation and AI-enabled annotation of trial data for information consistency.
- Expert review of trial and relevant clinical information categories
- Cross-referencing with official trial registries and external stakeholders
- Disclaimer for ARTICANZ
-
ARTICANZ (Annotated Registry of Trials in Cancer, Australia/NZ Edition; ARTificial Intelligence-enhanced CANcer trial information database) provides information about cancer drug trials in Australia and New Zealand as a free, open-information platform. Please note the following:
- Data Accuracy: While ARTICANZ strives to maintain accurate and up-to-date information through regular weekly updates and specialist review, we cannot guarantee the complete accuracy, reliability, or timeliness of all data. Information may change between updates.
- Not Medical Advice: The information provided by ARTICANZ is for informational purposes only and should not be considered medical advice. All clinical decisions should be made in consultation with qualified healthcare professionals.
- No Warranty: ARTICANZ is provided "as is" without any warranties, expressed or implied. We disclaim all liability for any damages arising from the use of or inability to use this platform.
- External Links: ARTICANZ may contain links to external websites. We are not responsible for the content or privacy practices of these sites.